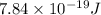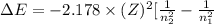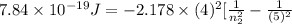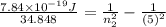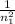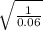Chemistry, 19.11.2019 03:31 kordejah348
One of the emission spectral lines for be31 has a wavelength of 253.4 nm for an electronic transition that begins in the state with n 5 5. what is the principal quantum number of the lower-energy state corresponding to this emission? (hint: the bohr model can be applied to one-electron ions. don’t forget the z factor: z 5 nuclear charge 5 atomic number.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
One of the emission spectral lines for be31 has a wavelength of 253.4 nm for an electronic transitio...
Questions

History, 01.03.2021 15:30

Chemistry, 01.03.2021 15:30

Mathematics, 01.03.2021 15:30






English, 01.03.2021 15:30



English, 01.03.2021 15:30

Mathematics, 01.03.2021 15:30

Business, 01.03.2021 15:30

Mathematics, 01.03.2021 15:30


Mathematics, 01.03.2021 15:30

Mathematics, 01.03.2021 15:40

Mathematics, 01.03.2021 15:40

English, 01.03.2021 15:40

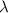 = 253.4 nm =
= 253.4 nm = 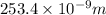 (as 1 nm =
(as 1 nm = 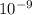 )
)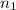 = 5,
= 5, 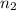 = ?
= ?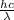
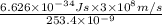
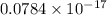 J
J