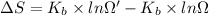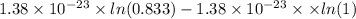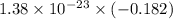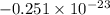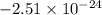Chemistry, 19.11.2019 03:31 wendii87wh
Agaseous system undergoes a change in temperature and volume. what is the entropy change for a particle in this system if the final number of microstates is 0.833 times that of the initial number of microstates? express your answer numerically in joules per kelvin per particle.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 08:30
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2
You know the right answer?
Agaseous system undergoes a change in temperature and volume. what is the entropy change for a parti...
Questions






Mathematics, 22.11.2020 02:30

English, 22.11.2020 02:30

Mathematics, 22.11.2020 02:30

Computers and Technology, 22.11.2020 02:30

English, 22.11.2020 02:30


Mathematics, 22.11.2020 02:30


Mathematics, 22.11.2020 02:30

English, 22.11.2020 02:30


Computers and Technology, 22.11.2020 02:30



Mathematics, 22.11.2020 02:30

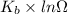
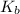 = Boltzmann constant
= Boltzmann constant = number of microstates
= number of microstates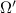 = 0.833
= 0.833