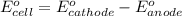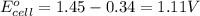Chemistry, 19.11.2019 03:31 mahadharun
Calculate the standard cell emf for the reaction: clo3−(aq)+3cu(s)+6h+(aq)→cl−(aq)+3c u2+(aq)+3h2o(l) pt is used as an inert electrode in contact with the clo3− and cl−. calculate the standard emf using data in appendix e and given the following: clo3−(aq)+6h+(aq)+6e−→cl−(aq)+3h2o( l); e∘=1.45 v express the emf to three significant figures with the appropriate units. v

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

You know the right answer?
Calculate the standard cell emf for the reaction: clo3−(aq)+3cu(s)+6h+(aq)→cl−(aq)+3c u2+(aq)+3h2o(...
Questions

Mathematics, 26.03.2021 16:20

Computers and Technology, 26.03.2021 16:20



Mathematics, 26.03.2021 16:20

Spanish, 26.03.2021 16:20




Mathematics, 26.03.2021 16:20


Mathematics, 26.03.2021 16:20


Mathematics, 26.03.2021 16:20

Mathematics, 26.03.2021 16:20


Mathematics, 26.03.2021 16:20


Mathematics, 26.03.2021 16:20

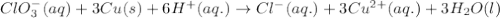
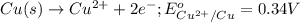 ( × 3)
( × 3)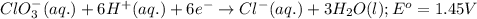
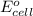 of the reaction, we use the equation:
of the reaction, we use the equation: