Chemistry, 19.11.2019 03:31 jdodger5165
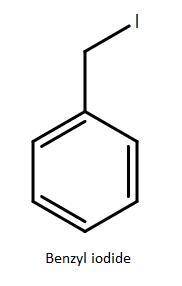
You synthesized barbituric acid in the highest yield to date and thus you were hired right off. now you have an idea for a barbiturate that will act as a sedative without the addictive side effects. your barbiturate replaces one of the methylene hydrogens of barbituric acid with a benzyl group. draw the skeletal structure of the alkyl iodide you would use to react with enolate of diethyl malonate.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
You synthesized barbituric acid in the highest yield to date and thus you were hired right off. now...
Questions

Physics, 13.04.2021 23:50




Mathematics, 13.04.2021 23:50

Mathematics, 13.04.2021 23:50

Physics, 13.04.2021 23:50



Mathematics, 13.04.2021 23:50

Computers and Technology, 13.04.2021 23:50


Arts, 13.04.2021 23:50

Mathematics, 13.04.2021 23:50

Mathematics, 13.04.2021 23:50


Mathematics, 13.04.2021 23:50

Mathematics, 13.04.2021 23:50

Mathematics, 13.04.2021 23:50

Chemistry, 13.04.2021 23:50