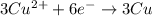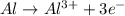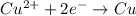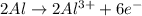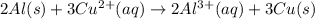When the following oxidation-reduction occurs, what is the balanced reduction half-reaction after the electrons in both half reactions are balanced? al (s) + cu2+ (aq) → al3+ (aq) + cu (s) view available hint(s) when the following oxidation-reduction occurs, what is the balanced reduction half-reaction after the electrons in both half reactions are balanced? al (s) + cu2+ (aq) → al3+ (aq) + cu (s) 2al (s) → 2al3+ (aq) + 6e− al (s) → al3+ (aq) + 3e– cu2+ (aq) + 2e− → cu (s) 3cu2+ (aq) + 6e− → 3cu (s)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
When the following oxidation-reduction occurs, what is the balanced reduction half-reaction after th...
Questions


SAT, 29.12.2021 14:00

SAT, 29.12.2021 14:00


History, 29.12.2021 14:00




SAT, 29.12.2021 14:00


Health, 29.12.2021 14:00

SAT, 29.12.2021 14:00



SAT, 29.12.2021 14:00


Business, 29.12.2021 14:00

Social Studies, 29.12.2021 14:00

SAT, 29.12.2021 14:00

Mathematics, 29.12.2021 14:00