
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. chlorine trifluoride is prepared by the reaction cl2 (g) 3f2 (g) ⟶ 2clf3 (g). write the equation that relates the rate expressions for this reaction in terms of the disappearance of cl2 and f2 and the formation of clf3.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile co...
Questions

History, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00

World Languages, 04.03.2021 20:00


Mathematics, 04.03.2021 20:00




Geography, 04.03.2021 20:00

English, 04.03.2021 20:00

English, 04.03.2021 20:00

Computers and Technology, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00


English, 04.03.2021 20:00

Social Studies, 04.03.2021 20:00


Advanced Placement (AP), 04.03.2021 20:00

![Rate=-\frac{d[Cl_2]}{dt}=-\frac{1}{3}\frac{d[F_2]}{dt}=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/d2692.png)
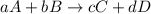
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0380/6825/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0380/6825/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0380/6825/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/d4b94.png)
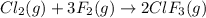
![\text{Rate of disappearance of }Cl_2=-\frac{d[Cl_2]}{dt}](/tpl/images/0380/6825/4403e.png)
![\text{Rate of disappearance of }F_2=-\frac{1}{3}\frac{d[F_2]}{dt}](/tpl/images/0380/6825/c24a1.png)
![\text{Rate of formation of }ClF_3=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/1400a.png)


