
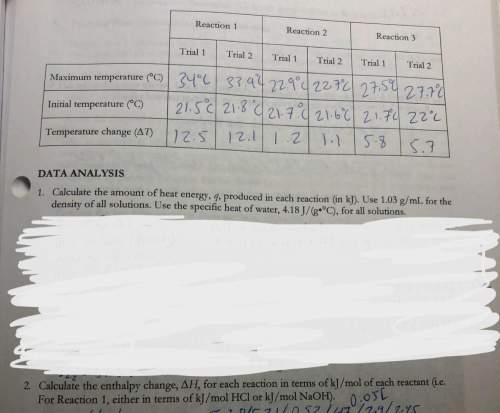
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for reaction three.
4. use hess’s law, and the accepted values of change of h in the pre-lab exercise to calculate the change in h for reaction 3. how does the accepted value compare to your experimental value?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

You know the right answer?
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for rea...
Questions





Mathematics, 26.06.2019 17:40

Mathematics, 26.06.2019 17:40



Computers and Technology, 26.06.2019 17:40













