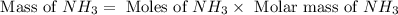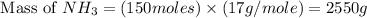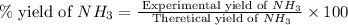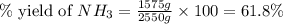Chemistry, 19.11.2019 06:31 jlbradley429
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with nitrogen, 1575 g of ammonia were produced. what is the percent yield of this reaction?
30.8%
61.8%
20.7%
41.5%
more information is needed to solve this problem.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1


Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with...
Questions

Mathematics, 25.05.2021 21:00



Mathematics, 25.05.2021 21:00


Mathematics, 25.05.2021 21:00

Mathematics, 25.05.2021 21:00


Mathematics, 25.05.2021 21:00

Mathematics, 25.05.2021 21:00


History, 25.05.2021 21:00

English, 25.05.2021 21:00

Mathematics, 25.05.2021 21:00

Business, 25.05.2021 21:00

English, 25.05.2021 21:00


Mathematics, 25.05.2021 21:00

Mathematics, 25.05.2021 21:00

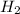 = 450 g
= 450 g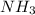 = 17 g/mole
= 17 g/mole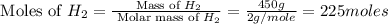
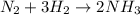
 moles of
moles of