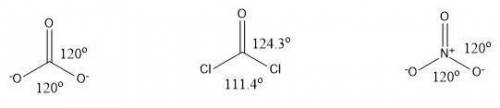
Consider the following molecules with trigonal planar geometry. carbonate (co32−) and nitrate (no3−) both exhibit resonance, whereas phosgene (cocl2) does not. given the information in the transition, and ignoring the simulation, predict the bond angles for each of the molecules in accordance to whether or not they exhibit resonance. drag the appropriate labels to their respective targets. view available hint(s)\

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Consider the following molecules with trigonal planar geometry. carbonate (co32−) and nitrate (no3−)...
Questions




World Languages, 28.06.2020 18:01

Mathematics, 28.06.2020 18:01


Biology, 28.06.2020 18:01


Mathematics, 28.06.2020 18:01


Mathematics, 28.06.2020 18:01

Mathematics, 28.06.2020 18:01

Mathematics, 28.06.2020 18:01


Mathematics, 28.06.2020 18:01


Mathematics, 28.06.2020 18:01

Biology, 28.06.2020 18:01

Mathematics, 28.06.2020 18:01