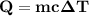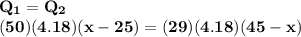Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 11:20
Match each state of matter with the statement that best describes it.
Answers: 1
You know the right answer?
A50.0 g sample of liquid water at 25.0 degree c is mixed with 29.0 g of water at 45 degree c. the fi...
Questions

Mathematics, 27.02.2021 02:10

Mathematics, 27.02.2021 02:10


Mathematics, 27.02.2021 02:10



Mathematics, 27.02.2021 02:10

Mathematics, 27.02.2021 02:10


English, 27.02.2021 02:10

Mathematics, 27.02.2021 02:10

History, 27.02.2021 02:10



English, 27.02.2021 02:10

SAT, 27.02.2021 02:10



Computers and Technology, 27.02.2021 02:10


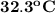 .
.