

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
Consider the complete combustion of glucose (c6h12o6) with o2 and calculate the moles of co2 produce...
Questions









Mathematics, 30.03.2020 22:01


English, 30.03.2020 22:01


Mathematics, 30.03.2020 22:01




Mathematics, 30.03.2020 22:01



English, 30.03.2020 22:01

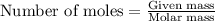
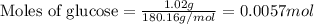

![37^oC=[37+273]K=310K](/tpl/images/0381/5720/20b22.png)
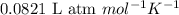
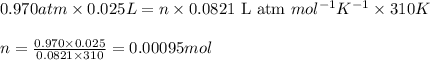

 of glucose
of glucose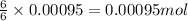 of carbon dioxide
of carbon dioxide

