
Chemistry, 19.11.2019 20:31 harmonywilliams3064
Which of the reactions are spontaneous (favorable)? a. dhap↽−−⇀glyceraldehyde-3-phosphateδ =3.8 kj/mol b. c2h4+h2−→−−rh(i)c2h6δ=−150.97 kj/mol c. glutamate+nad++h2o⟶nh+4+α-ketogluta rate+nadh+h+δ=3.7 kcal/mol d. c6h13o9p+atp⟶c6h14o12p2+adpδ=−14.2 kj/mol e. l -malate+nad+⟶oxaloacetate+nadh+h+δ= 29.7 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Which of the reactions are spontaneous (favorable)? a. dhap↽−−⇀glyceraldehyde-3-phosphateδ =3.8 kj/...
Questions


Mathematics, 23.05.2020 23:59



Physics, 23.05.2020 23:59






Biology, 23.05.2020 23:59


Mathematics, 23.05.2020 23:59


Spanish, 23.05.2020 23:59



History, 23.05.2020 23:59


Business, 23.05.2020 23:59

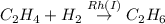 ; ΔG= −150.97 kJ/mol
; ΔG= −150.97 kJ/mol 

