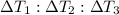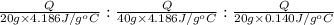Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity is 0.140 joules/gram degree celsius.
water and mercury are put into three identical bowls:
bowl a contains 20 grams of water.
bowl b contains 40 grams of water.
bowl c contains 20 grams of mercury.
the bowls start at the same temperature, and then the same amount of heat is added to each bowl. order the bowls from coolest to warmest, based on their final temperatures.
bowl a

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity...
Questions


Mathematics, 30.11.2020 01:00

English, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00



Mathematics, 30.11.2020 01:00



Mathematics, 30.11.2020 01:00


English, 30.11.2020 01:00



Mathematics, 30.11.2020 01:00


Mathematics, 30.11.2020 01:00

English, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00

Mathematics, 30.11.2020 01:00

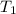
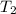
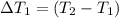
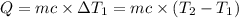
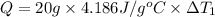
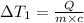
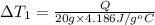 ..[1]
..[1]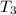
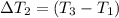
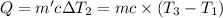
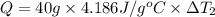
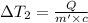
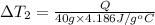 ..[2]
..[2]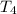
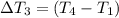
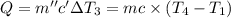
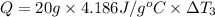
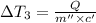
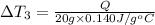 ..[3]
..[3]