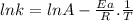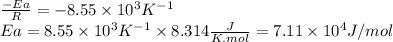The rate constant of a reaction is measured at different temperatures. a plot of the natural log of the rate constant as a function of the inverse of the temperature (in kelvins) yields a straight line with a slope of −8.55×103 k−1. what is the activation energy (ea) for the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
The rate constant of a reaction is measured at different temperatures. a plot of the natural log of...
Questions

Biology, 02.09.2019 02:30


Mathematics, 02.09.2019 02:30

Health, 02.09.2019 02:30

Social Studies, 02.09.2019 02:30

Mathematics, 02.09.2019 02:30

Mathematics, 02.09.2019 02:30

History, 02.09.2019 02:30

Mathematics, 02.09.2019 02:30





Arts, 02.09.2019 02:30




Chemistry, 02.09.2019 02:30