
Chemistry, 20.11.2019 00:31 brandon436
What is the ph of a buffer in which the concentration of benzoic acid, c6h5cooh, is 0.040 m and the concentration of sodium benzoate, nac6h5coo, is 0.015 m ? ka of c6h5cooh is 6.30 x 10-5

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
What is the ph of a buffer in which the concentration of benzoic acid, c6h5cooh, is 0.040 m and the...
Questions

Mathematics, 30.11.2021 23:40




World Languages, 30.11.2021 23:40


Health, 30.11.2021 23:40





Medicine, 30.11.2021 23:40

Mathematics, 30.11.2021 23:40

Arts, 30.11.2021 23:40




Computers and Technology, 30.11.2021 23:40

Chemistry, 30.11.2021 23:40

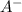 +
+ 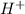
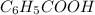 <=>
<=> 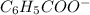 +
+ 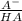
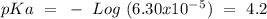
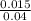 )
)


