
Chemistry, 20.11.2019 01:31 orlando19882000
You order a 16 oz glass of tea (where the mass of water is 474 grams) from a local restaurant. the tea is freshly brewed and has an initial temperature of 28.29 °c. you add ice to cool it. if the heat of fusion of ice is 6.020 kj/mol and each ice cube contains exactly 1 mol of water, how many ice cubes are necessary to cool the tea to 0.41 °c? the specific heat of the "tea" is 4.184 j/g*c.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
You order a 16 oz glass of tea (where the mass of water is 474 grams) from a local restaurant. the t...
Questions

Mathematics, 09.10.2021 03:00




Social Studies, 09.10.2021 03:00

Advanced Placement (AP), 09.10.2021 03:00



Biology, 09.10.2021 03:00




Mathematics, 09.10.2021 03:00

Mathematics, 09.10.2021 03:00

Mathematics, 09.10.2021 03:00


Physics, 09.10.2021 03:00

Mathematics, 09.10.2021 03:00


Biology, 09.10.2021 03:00

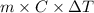
 = change in temperature =
= change in temperature = 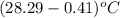 =
= 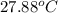
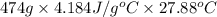
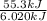
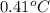 .
.

