

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
Which of the following transitions represent the emission of a photon with the largest energy?
Questions


Biology, 02.10.2019 05:40

Chemistry, 02.10.2019 05:40


Mathematics, 02.10.2019 05:40

Chemistry, 02.10.2019 05:40

Mathematics, 02.10.2019 05:40

English, 02.10.2019 05:40


Geography, 02.10.2019 05:40



History, 02.10.2019 05:40

History, 02.10.2019 05:40



Mathematics, 02.10.2019 05:40

History, 02.10.2019 05:40


Social Studies, 02.10.2019 05:40

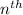 orbit is directly proportional to
orbit is directly proportional to 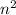 .Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to
.Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to  .So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).
.So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).

