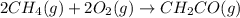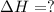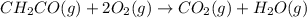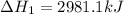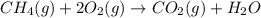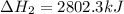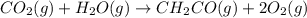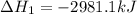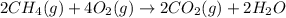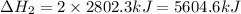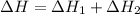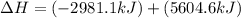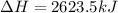Chemistry, 20.11.2019 06:31 treyhunt5362
The mea sured enthalpy change for burning ketene (ch2co) ch2co(g) 1 2 o2(g)88n 2 co2(g) 1 h2o(g) is dh1 5 2981.1 kj at 25°c. the enthalpy change for burning methane ch4(g) 1 2 o2(g)88n co2(g) 1 2 h2o(g) is dh2 5 2802.3 kj at 25°c. calculate the enthalpy change at 25°c for the reaction 2 ch4(g) 1 2 o2(g)88n ch2co(g) 1 3 h2o(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2


You know the right answer?
The mea sured enthalpy change for burning ketene (ch2co) ch2co(g) 1 2 o2(g)88n 2 co2(g) 1 h2o(g) is...
Questions

Mathematics, 15.02.2021 15:20

Mathematics, 15.02.2021 15:20

Computers and Technology, 15.02.2021 15:20

Mathematics, 15.02.2021 15:20


English, 15.02.2021 15:20

Biology, 15.02.2021 15:20

History, 15.02.2021 15:20

English, 15.02.2021 15:20

World Languages, 15.02.2021 15:20



Advanced Placement (AP), 15.02.2021 15:20

Social Studies, 15.02.2021 15:20


Mathematics, 15.02.2021 15:20


Mathematics, 15.02.2021 15:20

Mathematics, 15.02.2021 15:20

Mathematics, 15.02.2021 15:20