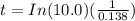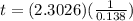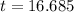Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2

Chemistry, 23.06.2019 11:50
How many moles of an ideal gas would occupy a 25.0 liter container when the temperature is 295 k and the pressure is 0.850 atm?
Answers: 2
You know the right answer?
The decay of the isotope iodine-131 is first-order with a rate constant of 0.138 d−1. all radioactiv...
Questions


Mathematics, 18.11.2020 20:10

Biology, 18.11.2020 20:10




Arts, 18.11.2020 20:10

Mathematics, 18.11.2020 20:10

Biology, 18.11.2020 20:10

Mathematics, 18.11.2020 20:10

Mathematics, 18.11.2020 20:10

Mathematics, 18.11.2020 20:10






Mathematics, 18.11.2020 20:10

Mathematics, 18.11.2020 20:10

![In(\frac{[A_{0}]}{[A]})=kt](/tpl/images/0383/1043/3c177.png)
![[A_{0}]](/tpl/images/0383/1043/747e3.png) is the initial concentration and
is the initial concentration and ![[A]](/tpl/images/0383/1043/6aa06.png) is the new concentration.
is the new concentration.![t=In(\frac{[A_{0}]}{[A]})/k](/tpl/images/0383/1043/f150b.png)
![t=In(\frac{[x]}{[0.10x]})(\frac{1}{0.138})](/tpl/images/0383/1043/fc51c.png)
![t=In(\frac{[1]}{[0.10]})(\frac{1}{0.138})](/tpl/images/0383/1043/2e7e2.png)