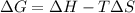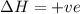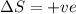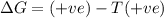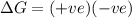Chemistry, 20.11.2019 20:31 Legoman29305
Consider a reaction that has a positive δh and a positive δs. which of the following statements is true? a) this reaction will be spontaneous only at high temperatures. b) this reaction will be spontaneous at all temperatures. c) this reaction will be nonspontaneous at all temperatures. d) this reaction will be nonspontaneous only at high temperatures. e) it is not possible to determine without more information.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Consider a reaction that has a positive δh and a positive δs. which of the following statements is t...
Questions




Mathematics, 30.11.2020 05:50

Mathematics, 30.11.2020 05:50

Chemistry, 30.11.2020 05:50

Computers and Technology, 30.11.2020 05:50






Mathematics, 30.11.2020 06:00

Mathematics, 30.11.2020 06:00


Mathematics, 30.11.2020 06:00

Mathematics, 30.11.2020 06:00


Advanced Placement (AP), 30.11.2020 06:00

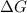 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous