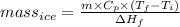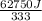Chemistry, 20.11.2019 20:31 jdsfdujfi1598
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitcher. if the tea is initially at 30.0°c and the ice cubes are initially at 0.0°c, how many grams of ice will still be present when the contents of the pitcher reach a final temperature? the tea is mostly water, so assume that it has the same density (1.0 g/ml), molar mass, heat capacity (75.3 j/k/mol), and heat of fusion (6.01 kj/mol) as pure water. the heat capacity of ice is 37.7 j/k/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitche...
Questions

Mathematics, 13.05.2021 19:50

Mathematics, 13.05.2021 19:50


Mathematics, 13.05.2021 19:50

Computers and Technology, 13.05.2021 19:50

Computers and Technology, 13.05.2021 19:50





Mathematics, 13.05.2021 19:50

English, 13.05.2021 19:50



Law, 13.05.2021 19:50


Mathematics, 13.05.2021 19:50

Mathematics, 13.05.2021 19:50


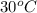 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K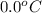 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K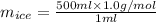
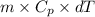
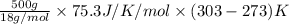
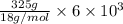
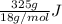 heat but we have 40774.95 J.
heat but we have 40774.95 J.