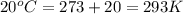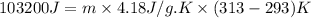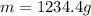Chemistry, 20.11.2019 20:31 bougiehairstudios
For many years drinking water has been cooled in hot climates by evaporating it from the surface of canvas bags or porous clay pots. how many grams of water can be cooled from 40 ∘c to 20 ∘c by the evaporation of 43 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj/g. the specific heat of water is 4.18 j/g⋅k.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
For many years drinking water has been cooled in hot climates by evaporating it from the surface of...
Questions

History, 27.04.2021 07:20



Mathematics, 27.04.2021 07:20


Mathematics, 27.04.2021 07:20

Mathematics, 27.04.2021 07:20

History, 27.04.2021 07:20



Computers and Technology, 27.04.2021 07:20



History, 27.04.2021 07:20

French, 27.04.2021 07:20


Mathematics, 27.04.2021 07:20



Mathematics, 27.04.2021 07:20

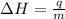
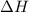 = enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g
= enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g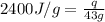
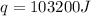
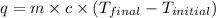
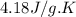
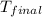 = final temperature =
= final temperature = 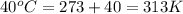
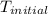 = initial temperature =
= initial temperature =