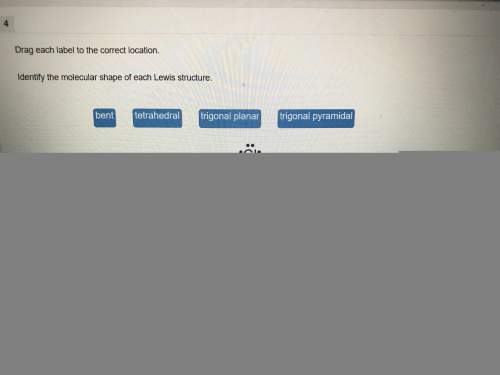
Chemistry, 20.11.2019 20:31 ashleybarrera2000
Calculate δg for the following reaction at 298.15 k 2 ag+(aq) + so42-(aq) ag2so4(s) for a solution in which the silver ion concentration is 0.0300 m and the sulfate ion concentration is 0.343 m. ksp(ag2so4) is 1.20 × 10-5 at 298.15 k.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Calculate δg for the following reaction at 298.15 k 2 ag+(aq) + so42-(aq) ag2so4(s) for a solution i...
Questions



Mathematics, 29.03.2020 03:02


Biology, 29.03.2020 03:03

Mathematics, 29.03.2020 03:03

History, 29.03.2020 03:03

History, 29.03.2020 03:03

Mathematics, 29.03.2020 03:03


Mathematics, 29.03.2020 03:03



English, 29.03.2020 03:03




Mathematics, 29.03.2020 03:03