
Chemistry, 20.11.2019 21:31 adriannacomrosenbark
Suppose of lead(ii) nitrate is dissolved in of a aqueous solution of ammonium sulfate. calculate the final molarity of lead(ii) cation in the solution. you can assume the volume of the solution doesn't change when the lead(ii) nitrate is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
Suppose of lead(ii) nitrate is dissolved in of a aqueous solution of ammonium sulfate. calculate the...
Questions


Mathematics, 09.01.2021 06:40


Mathematics, 09.01.2021 06:40

Mathematics, 09.01.2021 06:40

Engineering, 09.01.2021 06:40


Chemistry, 09.01.2021 06:40

Biology, 09.01.2021 06:40




English, 09.01.2021 06:40


Computers and Technology, 09.01.2021 06:40



Mathematics, 09.01.2021 06:40


Mathematics, 09.01.2021 06:40

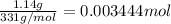
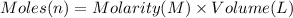
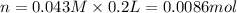
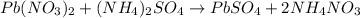
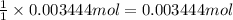 of ammonium sulfate
of ammonium sulfate![[Pb^{2+}]](/tpl/images/0383/3701/0acfd.png)
![[Pb^{2+}]=\frac{0.00 mol}{0.200 L}=0 M](/tpl/images/0383/3701/f1c13.png)


