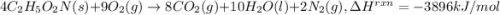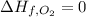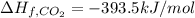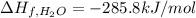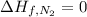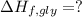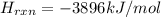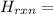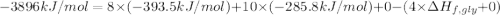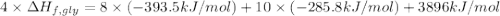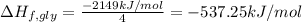Chemistry, 20.11.2019 21:31 clickbaitdxl
Glycine, c2h5o2n, is important for biological energy. the combustion reaction of glycine is described by the following thermochemical equation. 4c2h5o2n(s) + 9o2(g) → 8co2(g) + 10h2o(l) + 2n2(g) δh°rxn = –3896 kj/molwhat is the standard enthalpy of formation of solid glycine? –51.90 kj/mol–527.5 kj/mol–974.0 kj/mol–1502 kj/mol–2476 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

You know the right answer?
Glycine, c2h5o2n, is important for biological energy. the combustion reaction of glycine is describe...
Questions

Geography, 04.11.2019 06:31




History, 04.11.2019 06:31


Advanced Placement (AP), 04.11.2019 06:31




Mathematics, 04.11.2019 06:31

Mathematics, 04.11.2019 06:31

Mathematics, 04.11.2019 06:31







Social Studies, 04.11.2019 06:31