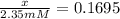Chemistry, 20.11.2019 22:31 elainnysanchez1541
The value of ? g�\' for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (2pg) is 4.4 kj/mol. if the concentration of 3-phosphoglycerate at equilibrium is 2.35 mm, what is the concentration of 2-phosphoglycerate? assume a temperature of 25.0 �c. the constant r = 8.3145 j/(mol�k)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
The value of ? g�\' for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (2pg) is 4.4 kj/m...
Questions


English, 06.04.2021 20:20


Mathematics, 06.04.2021 20:20


Mathematics, 06.04.2021 20:20


Mathematics, 06.04.2021 20:20


English, 06.04.2021 20:20

Computers and Technology, 06.04.2021 20:20




Mathematics, 06.04.2021 20:20

Geography, 06.04.2021 20:20

Mathematics, 06.04.2021 20:20

Business, 06.04.2021 20:20


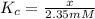
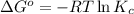
![4400 J/mol=-8.3145 J/mol K\times 298.15 K\times \ln [\frac{x}{2.35 mM}]](/tpl/images/0383/4523/5fe2b.png)
![\ln [\frac{x}{2.35 mM}]=\frac{4400 J/mol}{-8.3145 J/mol K\times 298.15 K}](/tpl/images/0383/4523/b97d3.png)
![\ln [\frac{x}{2.35 mM}]=-1.7750](/tpl/images/0383/4523/23145.png)