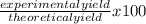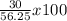Chemistry, 20.11.2019 23:31 jacobs5919
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + cl2 → c6h5cl + hcl , what is the percent yield of c6h5cl? 1. 50.0% 2. 53.4% 3. 69.4% 4. 13.2% 5. 76.9%

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3


Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + c...
Questions



English, 15.01.2020 05:31


English, 15.01.2020 05:31


Mathematics, 15.01.2020 05:31

Mathematics, 15.01.2020 05:31



History, 15.01.2020 05:31

Social Studies, 15.01.2020 05:31

Mathematics, 15.01.2020 05:31

History, 15.01.2020 05:31






Arts, 15.01.2020 05:31