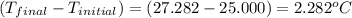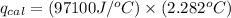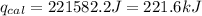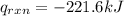Chemistry, 21.11.2019 01:31 Deadpool9609
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, according to the reaction below. the calorimeter (including the water) has a heat capacity of 97.1 kj/°c. c3h8(g) + 5 o2(g) 3 co2(g) + 4 h2o() (a) if the temperature rose from 25.000°c to 27.282°c, what is the heat of the reaction, qrxn?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, acc...
Questions

Mathematics, 21.01.2021 01:00



Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00







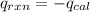
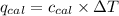
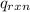 = heat released by the reaction = ?
= heat released by the reaction = ?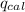 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter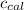 = specific heat of calorimeter =
= specific heat of calorimeter = 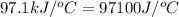
 = change in temperature =
= change in temperature =