
Chemistry, 21.11.2019 01:31 laurencollett4838
An arctic weather balloon is filled with 22.6l of helium gas inside a prep shed. the temperature inside the shed is 7°c. the balloon is then taken outside, where the temperature is 5°c. calculate the new volume of the balloon.?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
An arctic weather balloon is filled with 22.6l of helium gas inside a prep shed. the temperature ins...
Questions



Mathematics, 22.05.2021 19:50



Chemistry, 22.05.2021 19:50

Mathematics, 22.05.2021 19:50


Mathematics, 22.05.2021 19:50

English, 22.05.2021 19:50


Mathematics, 22.05.2021 19:50

Engineering, 22.05.2021 19:50




Mathematics, 22.05.2021 19:50



French, 22.05.2021 19:50

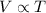

 = initial volume of gas = 22.6 L
= initial volume of gas = 22.6 L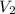 = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas =
= initial temperature of gas = 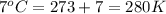
 = final temperature of gas =
= final temperature of gas = 




