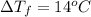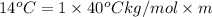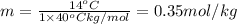Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Calculate the molality of isoborneol in the product if, a) the melting point of pure camphor is 179°...
Questions

Mathematics, 18.02.2021 19:40


Mathematics, 18.02.2021 19:40

Mathematics, 18.02.2021 19:40

Arts, 18.02.2021 19:40

Mathematics, 18.02.2021 19:40

Health, 18.02.2021 19:40

Mathematics, 18.02.2021 19:40


Biology, 18.02.2021 19:40




Mathematics, 18.02.2021 19:40



Mathematics, 18.02.2021 19:40


History, 18.02.2021 19:40

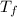 = 165°C
= 165°C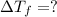
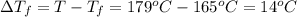
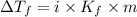
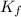 = The freezing point depression constant
= The freezing point depression constant