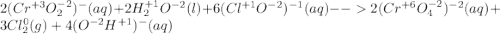Consider the following balanced redox reaction (do not include state of matter in your answers): 2cro2−(aq) + 2h2o(l) + 6clo−(aq) →2cro42−(aq) + 3cl2(g) + 4oh−(aq)(a) which species is being oxidized? (b) which species is being reduced? (c) which species is the oxidizing agent? (d) which species is the reducing agent? (e) from which species to which does electron transfer occur?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2



Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Consider the following balanced redox reaction (do not include state of matter in your answers): 2cr...
Questions

Mathematics, 26.07.2020 09:01

World Languages, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01


English, 26.07.2020 09:01




Social Studies, 26.07.2020 09:01

Mathematics, 26.07.2020 09:01


Mathematics, 26.07.2020 09:01






Mathematics, 26.07.2020 09:01