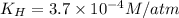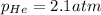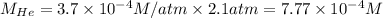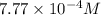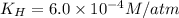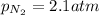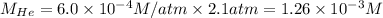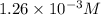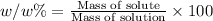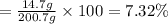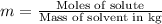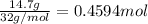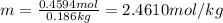Chemistry, 21.11.2019 02:31 josie17340
The henry's law constant for helium gas in water at 30 ∘c is 3.7×10−4m/atm; the constant for n2 at 30 ∘c is 6.0×10−4m/atm. a. if helium gas is present at 2.1 atm pressure, calculate the solubility of this gas. b. if n2 is present at 2.1 atm pressure, calculate the solubility of this gas.2. a solution is made containing 14.7 g of ch3oh in 186 g h2o. a. calculate the mass percent of ch3oh. b. calculate the molality of ch3oh.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
The henry's law constant for helium gas in water at 30 ∘c is 3.7×10−4m/atm; the constant for n2 at...
Questions


Chemistry, 23.08.2019 00:40





Mathematics, 23.08.2019 00:40

History, 23.08.2019 00:40

Chemistry, 23.08.2019 00:40

Mathematics, 23.08.2019 00:40




History, 23.08.2019 00:40






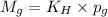
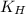 = Henry's constant
= Henry's constant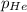 = partial pressure of gas
= partial pressure of gas