
Use the data given below to construct a born-haber cycle to determine the heat of formation of kcl. δ h°(kj) k(s) → k(g) 89 k(g) → k (g) e- 418 cl2(g) → 2 cl(g) 244 cl(g) e- → cl-(g) -349 kcl(s) → k (g) cl-(g) 717 use the data given below to construct a born-haber cycle to determine the heat of formation of kcl. h°(kj) k(s) → k(g) 89 k(g) → k (g) e- 418 cl2(g) → 2 cl(g) 244 cl(g) e- → cl-(g) -349 kcl(s) → k (g) cl-(g) 717 158 kj -1119 kj -997 kj 631 kj -437 kj

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 10:00
Which element forms a compound with chlorine with the general formula mci?
Answers: 1

Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions represents an exothermic reaction? nh3(g) + 12.0 kcal ½n2(g) + 3/2 h2(g) ch4 + 2o2 co2 + 2h2o + 212,800 cal c + 2s cs2, h = 27,550 cal c(graphite) c(diamond), h = 0.45 kcal 2h2o 2h2 + o2, h = +58 kcal
Answers: 1
You know the right answer?
Use the data given below to construct a born-haber cycle to determine the heat of formation of kcl....
Questions

Mathematics, 25.11.2021 14:00


Computers and Technology, 25.11.2021 14:00



Business, 25.11.2021 14:00








Business, 25.11.2021 14:00



Mathematics, 25.11.2021 14:00



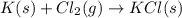
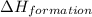 for this reaction.
for this reaction.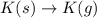 ,
, 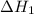 = 89 kJ
= 89 kJ
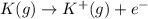 ,
, 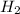 = 418 KJ
= 418 KJ
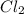 gas into chlorine atom
.
gas into chlorine atom
.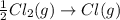 ,
, 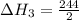 = 122 KJ
= 122 KJ
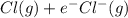 ,
, 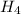 = -349 KJ
= -349 KJ
 ion and
ion and 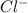 ion formed above to get KCl
.
ion formed above to get KCl
.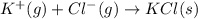 ,
, 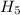 = -717 KJ
= -717 KJ



