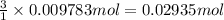The tungsten metal used for filaments in light bulbs is made by reaction of tungsten trioxide with hydrogen: wo3(s)+3h2(g)→w(s)+3h2o(g)
part a how many grams of tungsten trioxide must you start with to prepare 1.80 g of tungsten? (for wo3, mw = 231.8 amu.)
part b how many grams of hydrogen must you start with to prepare 1.80 g of tungsten?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
The tungsten metal used for filaments in light bulbs is made by reaction of tungsten trioxide with h...
Questions


Social Studies, 11.06.2021 22:40

Mathematics, 11.06.2021 22:40




Mathematics, 11.06.2021 22:40




Computers and Technology, 11.06.2021 22:40

Health, 11.06.2021 22:40





Mathematics, 11.06.2021 22:40

Mathematics, 11.06.2021 22:40

Mathematics, 11.06.2021 22:40

Mathematics, 11.06.2021 22:40

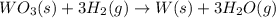
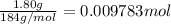
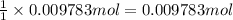 of tungsten trioxide
of tungsten trioxide