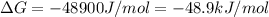Chemistry, 21.11.2019 04:31 alexreddin3127
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp (aq)+ h20 (l) -> adp (aq) + hpo4 (negative two overall charge) (aq).
for which ? g�rxn = �30.5 kj/mol at 37.0 �c and ph 7.0. calculate the value of ? grxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.80 mm, and [hpo42�] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions


Health, 28.09.2019 12:10


Mathematics, 28.09.2019 12:10


Chemistry, 28.09.2019 12:10

Biology, 28.09.2019 12:10






Mathematics, 28.09.2019 12:10

Physics, 28.09.2019 12:10



Mathematics, 28.09.2019 12:10

Chemistry, 28.09.2019 12:10


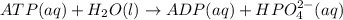
![[HPO_4^{2-}] = 5.0 mM=0.005 M](/tpl/images/0384/0544/f1ef4.png)
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}=\frac{0.0008 M\times 0.005 M}{0.005 M}=0.0008](/tpl/images/0384/0544/0fdb9.png)
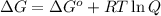
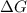 = Gibbs free energy at given conditions
= Gibbs free energy at given conditions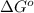 = Gibbs free energy at equilibrium=-30.5 kJ/mol
= Gibbs free energy at equilibrium=-30.5 kJ/mol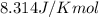
![37^oC=[273.15+37]K=310.15 K](/tpl/images/0384/0544/5ce7f.png)
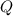 = reaction quotient at 37°C = 0.0008
= reaction quotient at 37°C = 0.0008![\Delta G=-30500 J/mol+(8.314J/Kmol)\times 310.15 K\times \ln [0.0008]](/tpl/images/0384/0544/d6332.png)