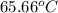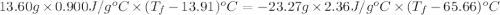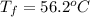Chemistry, 21.11.2019 05:31 tabocampos1414
A13.60-g block of solid aluminum at 13.91 °c is immersed in a 23.27-g pool of liquid ethylene glycol with a temperature of 65.66 °c. when thermal equilibrium is reached, what is the temperature of the aluminum and ethylene glycol? specific heat capacities: lead = 0.159 j/g °c; ethylene glycol = 2.36 j/g ° °c

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
A13.60-g block of solid aluminum at 13.91 °c is immersed in a 23.27-g pool of liquid ethylene glycol...
Questions



Biology, 30.08.2019 03:30

Social Studies, 30.08.2019 03:30

Mathematics, 30.08.2019 03:30

English, 30.08.2019 03:30


Social Studies, 30.08.2019 03:30


Geography, 30.08.2019 03:30



Computers and Technology, 30.08.2019 03:30

Computers and Technology, 30.08.2019 03:30




Biology, 30.08.2019 03:30

Mathematics, 30.08.2019 03:30

Computers and Technology, 30.08.2019 03:30

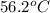
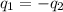
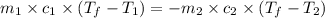
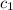 = specific heat of aluminum =
= specific heat of aluminum = 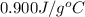
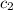 = specific heat of ethylene glycol =
= specific heat of ethylene glycol = 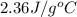
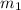 = mass of aluminum = 13.60 g
= mass of aluminum = 13.60 g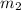 = mass of ethylene glycol = 23.27 g
= mass of ethylene glycol = 23.27 g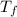 = final temperature of aluminum and ethylene glycol = ?
= final temperature of aluminum and ethylene glycol = ?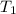 = initial temperature of aluminium =
= initial temperature of aluminium = 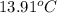
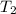 = initial temperature of ethylene glycol =
= initial temperature of ethylene glycol =