Chemistry, 21.11.2019 06:31 mraymundo025p0gpw9
Identify the following statements as true or false.1) all spontaneous reactions occur quickly.2) the energy of the universe is constant; the entropy of the universe increases.3) if a process increases the randomness of the particles of a system, the entropy of the system increases.4) all systems become more disordered spontaneously.5) all spontaneous processes release heat.6) both δ ssys and δ ssurr must equal zero at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
Identify the following statements as true or false.1) all spontaneous reactions occur quickly.2) the...
Questions


Mathematics, 20.05.2021 19:00


Engineering, 20.05.2021 19:00


Mathematics, 20.05.2021 19:00




Mathematics, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10

Biology, 20.05.2021 19:10


Advanced Placement (AP), 20.05.2021 19:10



Computers and Technology, 20.05.2021 19:10


Social Studies, 20.05.2021 19:10

English, 20.05.2021 19:10

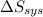 and
and 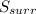 must equal zero at equilibrium.
must equal zero at equilibrium.