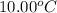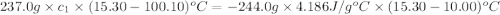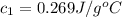A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup containing 244.0 g of water at 10.00 °c. if the final temperature of the water and metal in the cup is 15.30 °c, then what is the specific heat of molybdenum? (specific heat of water = 4.186 j/g-°c do not add the unit in the answer.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup co...
Questions

History, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31


English, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Biology, 28.01.2020 12:31

History, 28.01.2020 12:31

English, 28.01.2020 12:31

Business, 28.01.2020 12:31




Mathematics, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31


Mathematics, 28.01.2020 12:31

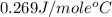
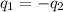
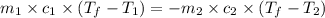
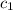 = specific heat of molybdenum metal = ?
= specific heat of molybdenum metal = ?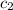 = specific heat of water =
= specific heat of water = 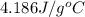
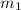 = mass of molybdenum metal = 237.0 g
= mass of molybdenum metal = 237.0 g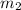 = mass of water = 244.0 g
= mass of water = 244.0 g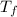 = final temperature of water and metal =
= final temperature of water and metal = 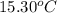
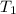 = initial temperature of molybdenum metal =
= initial temperature of molybdenum metal = 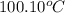
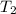 = initial temperature of water =
= initial temperature of water =