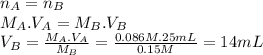Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m koh would need to be added to 25 ml of 0.086 m lactic acid to reach the equivalence point? (keep 2 significant figures) ml (b) at the equivalence point, would the aqueous solution be acidic, basic, or neutral? explain why at the equivalence point, the solution will be .. at this stage, all of the lactic acid in the solution will have reacted with the koh added, producing lactate lons (c3h503") and potassium ions (*) in the solution. the potassium ions will not affect the ph, but the lactate ions will make the solution -

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m ko...
Questions

Mathematics, 02.03.2021 02:20


Mathematics, 02.03.2021 02:20





Mathematics, 02.03.2021 02:20

Mathematics, 02.03.2021 02:20


Chemistry, 02.03.2021 02:20



Mathematics, 02.03.2021 02:20

Mathematics, 02.03.2021 02:20

Mathematics, 02.03.2021 02:20


Mathematics, 02.03.2021 02:20


English, 02.03.2021 02:20