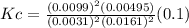Chemistry, 21.11.2019 09:31 thatonestudent2271
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equilibrium the concentration of no in the vessel is 0.161m. at equilibrium the vessel also contains n2, h2o, and h2. what is the value of the equilibrium constant for kc for the following reaction?
2h2 (g) + 2no(g) < —> 2h2o (g) + n2 (g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equ...
Questions

History, 15.09.2021 14:00


Chemistry, 15.09.2021 14:00



Mathematics, 15.09.2021 14:00




Mathematics, 15.09.2021 14:00


Social Studies, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00





Health, 15.09.2021 14:00


Mathematics, 15.09.2021 14:00

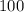 ml
ml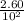 moles
moles moles
moles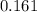 M
M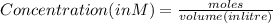
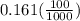
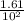 moles
moles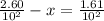
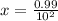
![Kc=\frac{[H2O]^2[N2]}{[H2]^2[NO]^2} (volume of vesselin litre)](/tpl/images/0384/4648/de870.png)