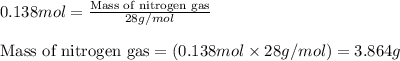Chemistry, 21.11.2019 20:31 rileyeddins1010
The rapid decomposition of sodium azide, nan3, to its elements is one of the reactions used to inflate airbags: 2 nan3 (s) 2 na (s) + 3 n2 (g) how many grams of n2 are produced from 6.00 g of nan

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
The rapid decomposition of sodium azide, nan3, to its elements is one of the reactions used to infla...
Questions

History, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00


History, 18.12.2020 01:00



Chemistry, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00


History, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00


Physics, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00

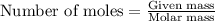 .....(1)
.....(1)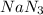 = 6.00 g
= 6.00 g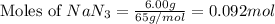
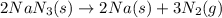
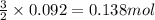 of nitrogen gas
of nitrogen gas