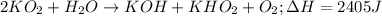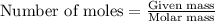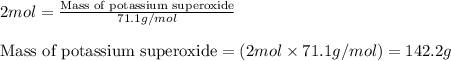Chemistry, 21.11.2019 20:31 coollid876
Potassium superoxide is a yellow paramagnetic solid that reacts with water according to the following balanced equation. calculate the mass of potassium superoxide required to produce 2405 j of heat.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Potassium superoxide is a yellow paramagnetic solid that reacts with water according to the followin...
Questions

Mathematics, 03.02.2020 14:57

Biology, 03.02.2020 14:57

Computers and Technology, 03.02.2020 14:57

Health, 03.02.2020 14:57


Mathematics, 03.02.2020 14:57


Mathematics, 03.02.2020 14:57

Geography, 03.02.2020 14:57

Social Studies, 03.02.2020 14:57

Physics, 03.02.2020 14:57


Mathematics, 03.02.2020 14:57

Health, 03.02.2020 14:57

History, 03.02.2020 14:57

English, 03.02.2020 14:58



Chemistry, 03.02.2020 14:58

History, 03.02.2020 14:58