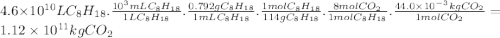Chemistry, 21.11.2019 20:31 jacksonyodell4184
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that the density of isooctane is 0.792 g/ml, what mass of co2 (in kilograms) is produced each year by the annual u. s. gasoline consumption of 4.6×1010l?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that...
Questions

World Languages, 13.06.2021 14:00


Physics, 13.06.2021 14:00

Mathematics, 13.06.2021 14:00

Mathematics, 13.06.2021 14:00

Mathematics, 13.06.2021 14:00

Mathematics, 13.06.2021 14:00

Physics, 13.06.2021 14:00

Physics, 13.06.2021 14:00

Social Studies, 13.06.2021 14:00


Arts, 13.06.2021 14:00

Biology, 13.06.2021 14:00



English, 13.06.2021 14:00

Mathematics, 13.06.2021 14:00

Mathematics, 13.06.2021 14:00


Mathematics, 13.06.2021 14:00