Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1
You know the right answer?
Aflask contains 0.25 mole of so2(g), 0.50 mole of ch4(g), and 0.50 mole of o2(g). the total pressure...
Questions


English, 15.08.2019 09:10



Mathematics, 15.08.2019 09:10

Physics, 15.08.2019 09:10


Mathematics, 15.08.2019 09:10






Mathematics, 15.08.2019 09:10

Biology, 15.08.2019 09:10



Mathematics, 15.08.2019 09:10

Mathematics, 15.08.2019 09:10

Mathematics, 15.08.2019 09:10

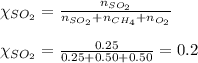
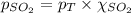
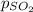 = partial pressure of sulfur dioxide gas
= partial pressure of sulfur dioxide gas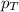 = total pressure = 800 mmHg
= total pressure = 800 mmHg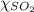 = mole fraction of sulfur dioxide = 0.2
= mole fraction of sulfur dioxide = 0.2