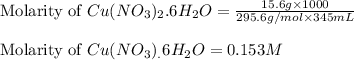Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 13:00
What did the experiments of scientists after john dalton reveal about his atomic theory?
Answers: 1
You know the right answer?
An aqueous solution is prepared by dissolving 15.6 g of cu(no3)2 ⋅ 6 h2o in water and diluting to 34...
Questions

Chemistry, 02.06.2021 14:00

Chemistry, 02.06.2021 14:00


Mathematics, 02.06.2021 14:00


History, 02.06.2021 14:00


Mathematics, 02.06.2021 14:00

Chemistry, 02.06.2021 14:00


English, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00




Mathematics, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00

Arts, 02.06.2021 14:00

Biology, 02.06.2021 14:00

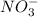 ions in the solution is 0.306 M
ions in the solution is 0.306 M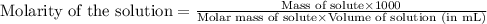
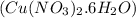 = 15.6 g
= 15.6 g