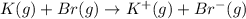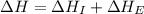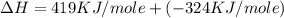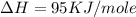Chemistry, 21.11.2019 21:31 shongmadi77
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following ionization energy (ie) and electron affinity (ea) values (hint: should one be negative for the reaction? ) ie ea k: 419 kj/mol 48 kj/mol br: 1140 kj/mol 324 kj/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following io...
Questions

Mathematics, 03.10.2021 14:00


Law, 03.10.2021 14:00


Mathematics, 03.10.2021 14:00


Mathematics, 03.10.2021 14:00

Chemistry, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00

Arts, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00

Chemistry, 03.10.2021 14:00



English, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00


Mathematics, 03.10.2021 14:00

Biology, 03.10.2021 14:00

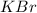 :
: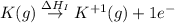
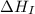 = ionization energy of potassium = 419 kJ/mol
= ionization energy of potassium = 419 kJ/mol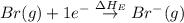
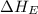 = electron affinity energy of bromine = -324 kJ/mol
= electron affinity energy of bromine = -324 kJ/mol