

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1


Chemistry, 23.06.2019 19:30
How has the scientific model of the atom changed over the centuries, and what new evidence led to the various changes in the model?
Answers: 1

Chemistry, 23.06.2019 22:30
Bernard is an excellent chef. however, his beef steak did not turn out well. bernard forgot that steak is tender and has less collagen, and he used the wrong cooking technique. which is the correct technique that bernard should have used? this subject is actually home ec they didnt have it when i had to pick so i chose anyone .
Answers: 1
You know the right answer?
Predict the sign of the entropy change of the system for each of the following reactions.
(a)...
(a)...
Questions

Mathematics, 04.10.2021 19:20

English, 04.10.2021 19:20


Mathematics, 04.10.2021 19:20


Mathematics, 04.10.2021 19:20




SAT, 04.10.2021 19:20

Geography, 04.10.2021 19:20

Mathematics, 04.10.2021 19:20

Business, 04.10.2021 19:20

Physics, 04.10.2021 19:20


Health, 04.10.2021 19:20

Mathematics, 04.10.2021 19:20



Biology, 04.10.2021 19:20

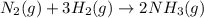
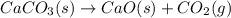
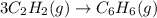
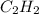 react to give 1 mole of gaseous
react to give 1 mole of gaseous 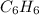 that means randomness become less that means the degree of disorderedness decreases. So, the entropy change will also decreases.
that means randomness become less that means the degree of disorderedness decreases. So, the entropy change will also decreases.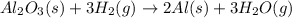
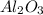 react to give 2 moles of solid aluminium that means randomness become more that means the degree of disorderedness increases. So, the entropy change will also increases.
react to give 2 moles of solid aluminium that means randomness become more that means the degree of disorderedness increases. So, the entropy change will also increases.

