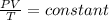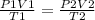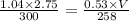Chemistry, 21.11.2019 22:31 darnellgee298
Ahelium balloon with a volume of 2.75 l and pressure of 1.04 atm and 27 °c flies in the sky and reaches an altitude where the temperature is -15 °c and the pressure is 0.530 atm. calculate the final volume of the balloon

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1


Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
Ahelium balloon with a volume of 2.75 l and pressure of 1.04 atm and 27 °c flies in the sky and reac...
Questions

Biology, 25.07.2019 01:00




Mathematics, 25.07.2019 01:00



History, 25.07.2019 01:00



Chemistry, 25.07.2019 01:00


English, 25.07.2019 01:00


Computers and Technology, 25.07.2019 01:00