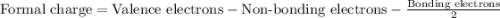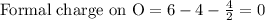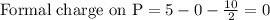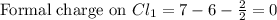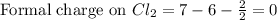Phosphorus forms a number of oxohalides, x3po, in which x may be a f, cl, or br atom. the most common of these, phosphoryl chloride, is obtained through the reaction 2pcl3(g)+o2(g)⟶2cl3po(g) draw the lewis structure for phosphoryl chloride. optimize formal charges.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Phosphorus forms a number of oxohalides, x3po, in which x may be a f, cl, or br atom. the most commo...
Questions


Chemistry, 19.07.2019 02:30

Physics, 19.07.2019 02:30

English, 19.07.2019 02:30




Social Studies, 19.07.2019 02:30






Biology, 19.07.2019 02:30






Mathematics, 19.07.2019 02:30

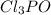 is shown below.
is shown below.