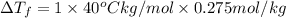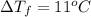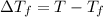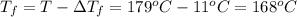Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Assume the molality of isoborneol in your product is 0.275 mol/kg. what is the melting point of your...
Questions



Social Studies, 29.07.2019 21:30




Biology, 29.07.2019 21:30



History, 29.07.2019 21:30

Social Studies, 29.07.2019 21:30

History, 29.07.2019 21:30

Chemistry, 29.07.2019 21:30




Biology, 29.07.2019 21:30


Chemistry, 29.07.2019 21:30

Business, 29.07.2019 21:30

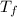 = ?
= ?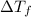
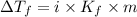
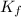 = The freezing point depression constant
= The freezing point depression constant